Abstract
Chimeric antigen receptor T (CAR T) therapy has revolutionized clinic outcomes of patients with hematologic malignancies, but therapeutic failure due to cancer resistance and relapse remains one of the major obstacles faced by CAR T cell therapy. We herein performed an unbiased CRISPR/Cas9 loss-of-function screening, which showed that enrichment of autophagy genes (ATG3, BECN1, RB1CC1 etc.) provided protection of cancer cells from CD19 CAR T cell-mediated cytotoxicity. Bulk RNA sequencing of clinical samples also suggested the clinical relevance of autophagy to therapeutic response and relapse in patients receiving CAR T cell therapy, indicating that autophagy may promote resistance to CAR-mediated cytotoxicity of B cell malignancies.
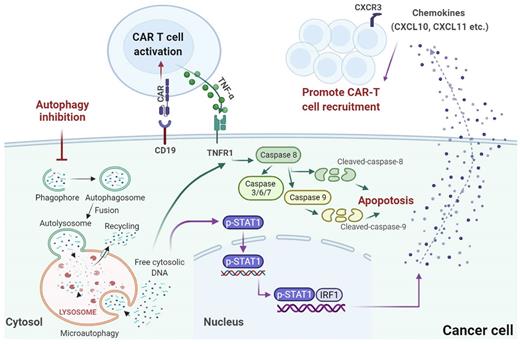
The addition of autophagy inhibitor (autophinib) during the co-culture with B cell malignancy cells significantly enhanced the killing effect of CD19 CAR T cells, whereas the addition of autophagy inducer (rapamycin) demonstrated the opposite effects. As expected, consistent results were observed upon knockout of key autophagy genes, further validating the protective role of autophagy. Pharmacological inhibition of autophagy induced caspase 8 and caspase 9 cleavage when co-cultured with CD19 CAR T cells, and the knockout of BECN1 and RB1CC1 also exhibited a substantial increase in the apoptosis. The induced apoptosis after autophagy inhibition could be obviously suppressed at the presence of TNFRSF1A knockout, which indicated that the protective effect of autophagy in the context of CAR T cell killing was mediated primarily through inhibition of TNF-α-induced apoptosis.
Despite the suppressed resistance to CD19 CAR T-driven killing observed in vitro cytotoxicity assays, we also observed consistent inhibition of tumor growth with the treatment of SAR405. Mice engrafted with RB1CC1-KO Raji-luc cells demonstrated attenuated resistance to CD19 CAR T cells in vivo. Remarkably, the increased infiltration of total CD3+ T and CD19 CAR T cells into Raji-luc lymphoma tumors treated with SAR405 was further confirmed by flow cytometry and immunohistochemistry staining. The cytokine profiling data identified that CXCL10 and CXCL11 were released, at least in part, from the tumor in response to autophagy inhibition. Using existing ChIP-seq datasets in ChIP-Atlas, we found that STAT1 and IRF1 had binding peaks in the promoter region of CXCL10 and CXCL11. Additionally, autophinib and SAR405 failed to induce CXCL10 and CXCL11 mRNA and protein expression in the absence of STAT1 or IRF1. Therefore, our data further validated the role of STAT1/IRF1 induced chemokine activation by autophagy targeting in reversing tumor immune escape.
In conclusion, this study offered a novel mechanism in which autophagy mediates cancer-intrinsic biology contributing to observed therapeutic failure during CAR T cell therapy, wherein both STAT1/IRF1-induced chemokine signaling activation and TNF-α induced apoptosis are involved (Figure 1). Our findings promote better understanding of the role of autophagy in cancer cell resistance to CAR T cells, which will help to identify crucial areas requiring further research to improve patient outcomes.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.